Chesapeake Bay Phosphorus Pollution Is Derived from Land-Based Sources
Don Boesch · | Applying Science |Donald F. Boesch, Walter Boynton, Jeffrey Cornwell, William Dennison, Michael Kemp, and Jeremy Testa
University of Maryland Center for Environmental Science
Bay Creates Its Own Phosphorus? Amidst the recent controversies regarding proposed requirements to reduce phosphorus runoff from agricultural soils a new article in the scientific journal Environmental Science & Technology (ES&T) was published in February 2015 with the title “Organic matter remineralization predominates phosphorus cycling in the mid-bay sediments in the Chesapeake Bay” [1]. Media claims suggesting that the research shows that runoff of phosphorus from land is not an important cause of impaired water quality, including the so-called dead zone, merit the closer examination provided here.
The article was published by researchers from the University of Delaware and two other institutions. These investigators employed isotopic, X-ray diffraction and spectroscopic methods to assess the characteristics of the phosphorus included in sediment cores collected at a site in the Chesapeake Bay east of Prince Frederick, Maryland. From their results they inferred the sources of the sediment phosphorus that leaves the sediment and re-enters the water column. They concluded that recycling of the phosphorus in organic matter—presumably produced by organisms living in the estuary—rather than in mineral matter was the predominant pathway to support primary production in the Chesapeake Bay.
A University of Delaware UDaily news media item [2] posted at the time of publication of the journal article indicated that the study demonstrated for the first time that recycling of phosphorus from organic matter is the predominant pathway for phosphorus cycling. The posting goes beyond what is stated in the article to say that while some believe that hypoxia, or the dead zone, can be attributed to remobilization of phosphorus entering from terrestrial or atmospheric sources settling to the sediment and then mobilizing to bottom water, this “research suggests that the problem lies in organic matter remineralization.” The popular press carried this even further, with the Delmarva Farmer editor writing that the scientists were “suggesting the Chesapeake Bay’s principal problem with phosphorus is not what is coming off the land around it but what it is creating within its own waters.” “Research suggests Bay creates its own phosphorus” states the headline.
Importance of Organic Remineralization. While the article may be the first time that the importance of organic remineralization has been demonstrated using such geochemical methods and inferences, its importance has long been recognized and demonstrated by Chesapeake Bay ecologists and chemists. In the middle estuary, including the site where cores analyzed in the study were collected, most of phosphorus deposited onto bottom sediments has been shown to be derived from organic matter produced in the surface waters of the Bay. Furthermore, extensive measurements of the phosphorus and nitrogen flux from sediments into the water column have been reported in the literature for over 30 years. Unlike the present geochemical study that is based on archived frozen cores collected at one site at one time (July), these published flux measurements were made at numerous sites covering a range of salinity and sediment types along the estuarine gradient and over annual seasons and many years, including drought and flood years. Moreover, these deposition and sediment flux estimates are based on rates actually measured in the field or using freshly collected cores, rather than inferred from geochemical profiles.
The recycling of phosphorus from bottom sediments, including the deposition and remineralization of organic matter, is in fact well-represented by equations and parameters included in the Chesapeake Bay Program’s numerical model of the estuary that was used in determining the Total Maximum Daily Load on which required nutrient load reductions are based. Therefore, the important role of organic remineralization of phosphorus is not novel to either Bay science or management.![The 78 Chesapeake Bay segments used in the Estuarine Hydrodynamic and Water Quality Models (left) and tracer simulation model showing Susquehanna river plume (right). Diagram from Chesapeake Bay Environmental Models, CBP and IAN [pdf] The 78 Chesapeake Bay segments used in the Estuarine Hydrodynamic and Water Quality Models (left) and tracer simulation model](/site/assets/files/2448/model-500x362.png)
Fate of Particulate Phosphorus. In addition to wastes directly discharged into the rivers and tidal waters, which have been dramatically reduced through improvements in wastewater treatment, rivers and streams deliver dissolved phosphate derived from agricultural soils and urban surfaces. These rivers and streams also carry large quantities of particulate phosphorus, including: (1) phosphorus incorporated into the structure of minerals eroded from parent rocks, (2) phosphorus that is co-precipitated within aquatic systems, and (3) phosphorus that is adsorbed onto suspended soil or sediment particles. Adsorption is particularly facilitated when the particles contain oxidized iron, to which phosphate has a strong attraction. A portion of this particulate phosphorus is deposited mainly in the upper end of the Chesapeake Bay and its tidal rivers, but relatively little of the riverine particulates are likely to be deposited where the sediment cores were collected over 100 miles below the mouth of the Susquehanna, although there may be more local inputs due to shoreline erosion. Particulate phosphorus, either in suspension or deposited, can be mobilized as dissolved phosphate thus becoming available to phytoplankton and bacteria. However, the ES&T study cannot resolve the extent of this mobilization along the full estuarine gradient as it is based on one site in the mid-reaches of the estuary. Furthermore, the study does not differentiate among the three forms of particulate phosphorus mentioned above.
The UDaily media release states: “The research showed that the iron oxide dissolution seems to be a minor source of the phosphorus in the area of the bay they studied. This means that a bulk of land driven phosphorus remains in sediment and is not active anymore.” However, this finding might be aliased by the reliance on cores collected only during July. Previous studies nearby have shown that substantial quantities of iron-bound phosphorus are released from bottom sediments when bottom waters become seriously hypoxic, typically by June or before. The lack of oxygen allows sulfide produced by anaerobic bacteria in bottom sediments to permeate to the sediment surface. Sulfide has an even stronger attraction to the iron on sediment particles than phosphate, which then is desorbed and fluxes into the water column. This has been observed many times in field measurements and laboratory experiments [4]. That release of adsorbed phosphorus would have already occurred when these cores were collected in July.
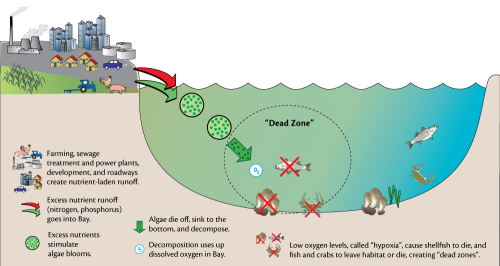
Effects on Hypoxia. The release of phosphorus from sediments does not directly sustain or refuel hypoxia as intimated in the ES&T article and stated in the press release. Rather, the phosphorus must be mixed into lighted surface waters and fuel another round of organic matter production [5]. The decomposition of sinking fresh organic matter can then deplete oxygen concentrations in bottom waters. However, during the time when hypoxia is most intense the production tends to be limited by the availability of nitrogen rather than phosphorus. Rather than intensifying hypoxia that summer, the excess phosphorus might play a role in supporting the beginning of the production cycle early the next spring when production is phosphorus-limited [6][7]. But there are very low fluxes of phosphate from sediments at that time of the year, suggesting that phosphorus inputs from watershed sources are probably more important.
Long-Term Consequences. Despite what is implied in the media reports, the eutrophication and associated impairment of water quality in Chesapeake Bay is ultimately fueled by land-based inputs of phosphorus and nitrogen. Unlike reactive nitrogen, which can be newly fixed in aquatic ecosystems, phosphorus is conserved—it can be flushed into or out of the estuary or stored in bottom sediments, but it cannot be created in the estuary. Rather, organic matter remineralization is well-known as an amplifier in the vicious cycle of eutrophication, allowing the primary producers of organic matter to reuse the phosphorous several times before being either deposited or flushed out of the estuary.
How long will the lingering effects of this remineralization last and thus delay the improvements to water quality from reducing the source of inputs? That’s a very pertinent question. Both the computer models and the experimental measures on which they are based suggest that this lag time is rather short, certainly not more than two or three years, a far cry from the 10 to 50 years suggested by one of the researchers in the UDaily media release. Thus, field, experimental and modeling results all clearly indicate that decreasing external inputs of both nitrogen and phosphorus, as call for to meet the TMDL, will lead to improved water and habitat quality and that Bay responses to these reductions should be fairly rapid rather than very slow. This is consistent with experience in tidally flushed coastal systems elsewhere [8][9] as well as at locations in the Chesapeake estuary where wastewater nitrogen and phosphorus loads were significantly reduced [10].
Citations:
[1] Joshi, S.R.., R.K. Kukkadapu, S.J. Burdige, M.E. Bowden, D.L. Sparks, and D.P. Jaisi. 2015. Organic matter remineralization predominates phosphorus cycling in the mid-bay sediments in the Chesapeake Bay. Environmental Science & Technology DOI: 10.1021/es5059617
[2] Thomas, Adam. 2015. Remineralization: UD-led study suggests new pathway for phosphorus cycling in Chesapeake Bay. UDaily, University of Delware, February 19, 2015.
[3] Boynton, W.R., J.H. Garber, R. Summers and W.M. Kemp. 1995. Inputs, transformations, and transport of nitrogen and phosphorus in Chesapeake Bay and selected tributaries. Estuaries 18:285-314.
[4] Boynton, W.R. 2000. Impact of nutrient inflows on Chesapeake Bay, p. 23-40. In: A.N. Sharpley (ed). Agriculture and Phosphorus Management: the Chesapeake Bay. Lewis Publishers, Boca Raton, Florida [pdf]
[5] Kemp, W.M. and 17 others. 2015. Eutrophication of Chesapeake Bay: historical trends and ecological interactions. Marine Ecology Progress Series 303:1-29.
[6] Fisher, T.R., E.R. Peele, J.A. Ammerman, and L.W. Harding. 1992. Nutrient limitation of phytoplankton in Chesapeake Bay. Marine Ecology Progress Series 82:51-63. [pdf]
[7] D’Elia, C.F., J.G. Sanders and W.R. Boynton. 1986. Nutrient enrichment studies in a coastal plain estuary: phytoplankton growth in large-scale, continuous flow cultures. Canadian Journal of Fisheries and Aquatic Sciences 43:397-406.
[8] Tucker, J., A.E. Giblin, C..S. Hopkinson and S.W. Kelsey. 2014. Response of benthic metabolism and nutrient cycling to reductions in wasterwater loading to Boston Harbor, USA. Estuarine, Coastal and Shelf Science 151:54-68.
[9] Valdemarsen, T., C.O. Quintana, M.R. Flindt and E. Kristensen. 2015. Organic N and P in eutrophic fjord sediments—rates of mineralization and consequences for internal nutrient loading. Biogeosciences 12:1765-1779.
[10] Lyerly CM, Hernandez Cordero AL, Foreman KL, Phillips SW, Dennison WC. 2014. New Insights: Science-based evidence of water quality improvements, challenges, and opportunities in the Chesapeake. IAN Press, Cambridge, MD.