Celebrating 100 Years of Industrial Nitrogen Fixation
Bill Nuttle ·People are part of a hybrid socio-environmental ecosystem.
The debate over whether people should start geoengineering the atmosphere in order to prevent the worst effects of global warming ignores one essential fact - we already are geoengineering the atmosphere. Geoengineering is the deliberate effort to manipulate processes that control conditions in the atmosphere on a global scale. Last month, March 2013, marked the centennial anniversary of the invention of the Haber-Bosch process, an industrial process for “fixing” nitrogen by producing ammonia from nitrogen in the atmosphere. Using the Haber-Bosch process, people now have a large influence over the global nitrogen cycle.
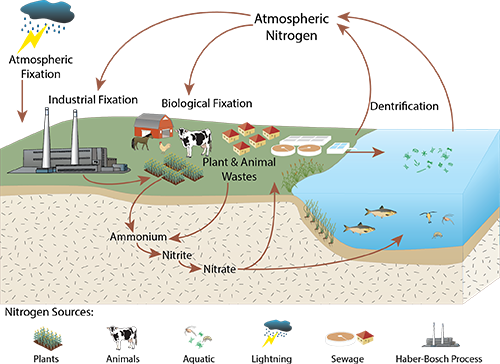
Earth is a planet that is mostly covered by water and surrounded by an atmosphere that is mostly composed of nitrogen. Yet, it is one of the ironies of nature that so much of the life here is organized around a constant struggle to secure sufficient water and nitrogen. Nitrogen is, of course, an essential plant nutrient and a key element in the protein molecules that make up our bodies. Just as the water of the oceans is unfit for us to drink, most plants cannot use the dinitrogen gas, N2, that makes up 78% of the atmosphere. Until the 20th century, plants and animals had to depend almost entirely on microbes to scavenge nitrogen from the atmosphere. A few microorganisms have the ability to “fix” dinitrogen gas, converting nitrogen from the atmosphere into a form that plants can use; lightning is also responsible for a small amount of nitrogen fixation.
N2 + 3H2 → 2NH3 (ΔH = -92.22kJ·mol-1)
The Haber-Bosch process converts nitrogen gas from air into a form that can be used for fertilizer.
The invention of the Haber-Bosch process changed all of that. The Haber-Bosch process is used by the chemical industry to combine dinitrogen gas with hydrogen under high pressure and temperature, in the presence of a catalyst, to produce ammonia, which can be used to produce a plant fertilizer. The first factory to use the Haber-Bosch process began operating 100 years ago, in 1913. As with the industrial desalinization of sea water to produce drinking water, the Haber-Bosch process frees people from the struggle to find sufficient quantities of nitrogen in a form that can be used to grow our food. The immediate costs of this freedom are in the construction of the chemical factories and in the energy required to run them, which are relatively small compared to its direct benefits. Clearly, people benefit from the use of the Haber-Bosch process; some estimate that the human population would be only half what it is today without this industrial boost to the nitrogen cycle. However, we are also plagued by its unintended consequences.
Since throwing the switch on the Haber-Bosch process people have taken a major role in the global nitrogen cycle that controls the distribution and movement of nitrogen throughout the environment. Today, the chemical industry converts more atmospheric nitrogen into nutrient form than the combined action of all the nitrogen-fixing microbes over the entire globe. If desalinization had a similar impact on the hydrologic cycle, by comparison, then we would have more than doubled the flow of all the world’s rivers into the sea.
Can we build a better socio-environmental ecosystem?
We may control half of the global nitrogen cycle, but let’s face the fact that we are not doing a very good job of it. On the input side, we are running a huge industrial process that uses the Haber-Bosch process to scavenge nitrogen from the air and artificially convert it into fertilizer to grow our food. On the output side, we are running a huge industrial process to collect nutrient-rich human waste and convert the nitrogen it contains back into atmospheric dinitrogen gas. And, the environment suffers from nutrients leaking out at both ends – too much fertilizer is produced and wasted in its application, and not all the nutrients are removed from human waste before it is discharged to the environment.
The Chesapeake Bay suffers from an excess of the “fixed” forms of nitrogen, as fertilizer, as nitrate and nitrite from pollutants in the air, and as a component of animal and human waste. The strategy to restore the bay by putting it on a “pollution diet” focuses on identifying sources for these substances and taking various actions in the watershed to keep them from getting into the water. However, there is more to it than just that. What people do and the choices we make have global consequences, such as changing the climate, that also affects the Bay. People are now part of a hybrid socio-environmental ecosystem that connects us with the environment on a global scale, as well as locally. It's important to remind ourselves of this fact, and that's why we remember the anniversary of the Haber-Bosch process.